Dry Powder Inhaler Devices
Mediplas: A certified and trusted leader in Dry Powder Inhaler (DPI) manufacturing, specializing in large-scale production with exceptional quality standards.

Mediplas: A certified and trusted leader in Dry Powder Inhaler (DPI) manufacturing, specializing in large-scale production with exceptional quality standards.

The manufacturing process for DPI Aerohaler Device consists of the following steps

The raw material is received from logistics channel and stored in the raw material store
The Material is Heated and Subsequently Processed Through the Injection Molding Technique to Achieve Desired Shapes
The components are produces from injection molding machines with precision molds and validated process parameters
The inspection during the injection molding process is conduction for product confirmation
The in-process inspection is conducted for monitoring process parameters and produced components
Each Component Created Through Injection Molding is Carefully Assembled and Subjected to Thorough Inspection
After Each Device Is Fully Assembled, It Is Individually Packed in Bags to Maintain Quality and Protection Until Delivery.
Each packed device is transferred to a unit carton with a leaflet providing usage instructions
The Unit Carton Is Carefully Sealed Using Labels on Both Ends to Ensure Secure Closure and Protect the Contents During Transport.
These packed unit cartons are shifted to a master carton which is labelled and ready for dispatch




| S. # | Component | Material |
| 1 | Cap | ABS |
| 2 | Mouth Piece | ABS |
| 3 | Capsule Chamber | ABS |
| 4 | Lid | ABS |
| 5 | Slider (with pins) | ABS + SS |
| 6 | Spring | SS |
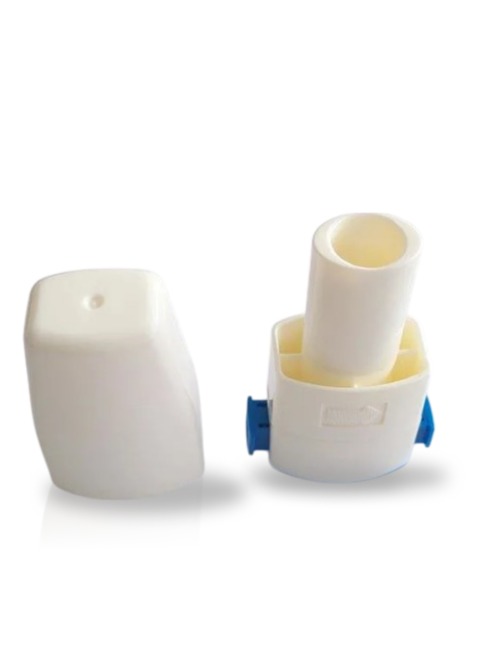
| Item Name | DPI, Aerohaler |
| Item Code | 01080003 |
| Manufacturer | Mediplas Innovations Pvt. Ltd. |
| Address | Plot LE 15, A1 Korangi Creek Industrial Park, Karachi |
| Postal Code | 75190 |
| Telephone # | 021-38893878-82 |
In combination with a drug-containing capsule of size 3, the drug is quantitatively inhaled through
the oral cavity by the negative pressure formed through patient’s inhalation.

To open, hold the device at the capsule chamber with one hand and remove the cap.
Rotate the mouth piece by holding the capsule chamber from one had and moving the mouth
piece in the direction of arrow indicated in mouth piece.
Insert the capsule into the chamber horizontally.
Revert the mouth piece and close the device with a click sound.
Press the sliders (on both sides) to puncture the capsule.
Breathe out fully through mouth. Position the mouthpiece between teeth and close the lips
tightly around it. Sit or stand upright, keep your head straight, and breathe in through the
mouth rapidly and deeply. When done correctly, you will hear the capsule vibrating inside the
device.
Remove the device from your mouth and hold your breath for about 10 seconds or for as long as
it is comfortable. Breathe out normally. In case some powder remains in the chamber, repeat
step 5. Ensure that no powder remains in the chamber.
After every use, open the mouthpiece and discard the empty capsule.
Close the mouth piece, put on the cap and store the device in the zip lock provided

Open the mouthpiece of the device.
If there is an empty capsule in the chamber, remove it.
Rinse the mouthpiece and chamber in clean running water.
Shake well to remove excess water and leave it to air dry.
Clean the device at least once a week.
Never use the device when it is wet.
Do not push any cloth or instrument into the mouthpiece of the device as it may cause damage.
Keep the capsule bottle tightly closed to avoid exposure to moisture.
You can continue to use your device daily for up to 6 months and then discard it and replace
with a new one.